galvanized steel is the kind of steel that is usually formed in sheets and the material is coated with zinc to be protected against corrosion. The thickness of these products determines their gauge. Galvanized iron is used in the production of a vast number of different objects, ranging from nails and bolts to bridges and structural components. In point of fact, galvanized iron has been around for centuries, and some of its earliest recorded usage date all the way back to the 17th century. However, over the course of the past few years, it has emerged as a credible substitute for conventional iron, in addition to a number of other metals and alloys. However, if you are not familiar with it, you may be asking what galvanized iron is and how it is different from regular iron. Galvanized iron is covered in greater detail in the following section. Iron that has been galvanized has effectively had a layer of zinc applied to its surface so that it is protected from the elements. Iron is prone to deterioration brought on by exposure to the elements. Iron, for instance, will rust and corrode when it is subjected to oxygen and moisture in the air. The presence of rust and corrosion can eat away at the iron over the course of time, putting the iron's structural integrity in jeopardy. Galvanization, which offers a straightforward and efficient method to protect iron from deterioration caused by environmental factors such as this one, is quite fortunate. Iron that has been galvanized has had an additional layer of zinc applied to it, whereas iron that has not been galvanized is made up solely of iron. The process of galvanizing iron involves immersing the iron in a bath of liquid zinc. Through the use of heat, the zinc is brought from its solid form into its liquid condition. The iron is next cooled after being submerged in a bath of molten zinc, which is followed by another dipping step. 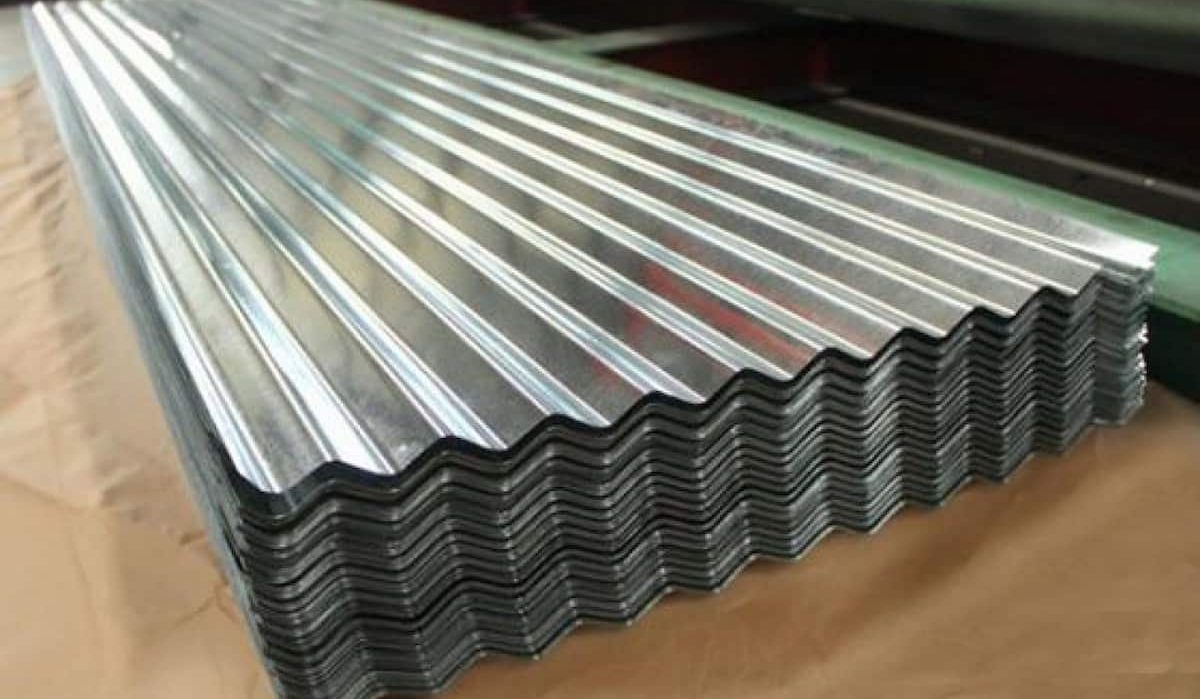 When the zinc reaches room temperature, it will begin to solidify and will eventually form a protective shell over the surface of the iron. The only difference between galvanized iron and normal iron is that galvanized iron has a layer of zinc applied to its surface. A layer of zinc has been put on top of the iron, which helps to protect it against rust and corrosion. If it is not there, the iron will be vulnerable to the moisture and oxygen that are present in the environment around it. In the absence of any preventative measures, this will result in the oxidation of the iron due to a chemical reaction. The iron will corrode and rust as a result of the oxidation process. The hop-dip process is just one of the several methods that can be used to manufacture galvanized iron. Other methods include: In the hot-dip process, zinc is deposited onto the surface of iron by first dipping the iron into molten zinc, and then drawing the iron out of the zinc. It is by far the most common approach employed when producing galvanized iron. There are two primary ways to galvanize metal: the hot-dip method and the cold-galvanization method. The process of brushing a layer of cold zinc over the surface of iron is what's known as cold galvanizing.
When the zinc reaches room temperature, it will begin to solidify and will eventually form a protective shell over the surface of the iron. The only difference between galvanized iron and normal iron is that galvanized iron has a layer of zinc applied to its surface. A layer of zinc has been put on top of the iron, which helps to protect it against rust and corrosion. If it is not there, the iron will be vulnerable to the moisture and oxygen that are present in the environment around it. In the absence of any preventative measures, this will result in the oxidation of the iron due to a chemical reaction. The iron will corrode and rust as a result of the oxidation process. The hop-dip process is just one of the several methods that can be used to manufacture galvanized iron. Other methods include: In the hot-dip process, zinc is deposited onto the surface of iron by first dipping the iron into molten zinc, and then drawing the iron out of the zinc. It is by far the most common approach employed when producing galvanized iron. There are two primary ways to galvanize metal: the hot-dip method and the cold-galvanization method. The process of brushing a layer of cold zinc over the surface of iron is what's known as cold galvanizing. 
galvanized iron
The process of galvanizing involves coating iron or steel with zinc to increase the base material's resistance to corrosion. In 1837, the technique for galvanizing sheet iron was concurrently developed in France and England. Both of these techniques used a "hot dipping" procedure to apply zinc to sheet iron. Early galvanized metals were hand-dipped, similar to tinplate. The majority of steel and iron in use today has been electroplated. The most popular techniques for putting zinc protective coatings on iron and steel are as follows: Hot-dip Galvanizing is the process of submerging iron or steel in molten zinc after the base metal's surface has been thoroughly cleaned. This procedure results in a reasonably thick zinc coating that crystallizes into the spangles surface pattern. Between the inner surface of the zinc coating and the iron or steel, a multilayered structure of iron- or steel-zinc alloys is created during the process. If the iron or steel element is bent, these middle layers, which have a tendency to be hard and brittle, could peel or flake. Iron or steel is submerged in an electrolyte, such as a zinc sulfate or cyanide solution, during electrogalvanizing. The iron or steel surface is coated with pure zinc thanks to electrolytic activity. Advantages: Using this method, the coating's thickness can be precisely regulated. Limitations: This technique typically cannot provide the thick coatings offered by the hot-dip galvanizing procedure. Sherardizing is the process of enclosing an iron or steel component that has been carefully cleaned in a space devoid of air and covered in metallic zinc dust. A thin, zinc alloy coating is then generated by heating the architectural component. Advantages: The coating will follow the element's settings. Limitations: This method is typically only effective with small things.  Metallic spraying is when molten zinc is applied in a fine mist to a clean iron or steel part. To create an alloy, the coating can then be heated and fused with the surface of the iron or steel. Benefits: Coating produces products that are less fragile than those made by some of the other techniques. On bending, the coating won't peel or flake. Limitations: As corrosion agents fill in the pores, the covering becomes more porous and eventually impermeable. Painting: As a protective coating, paint incorporating zinc dust pigments may be used. Advantages Advantages In situ painting is a possibility. Limitations: Compared to the other zinc coating techniques mentioned above, this one is less successful. Pure zinc, galvanized steel, or iron do not take paint well. When paint on galvanized iron and steel peels, it typically removes completely, leaving a spotless metal surface exposed. It might be challenging to tell whether sheet metal components are made of zinc, galvanized iron, or steel if they are well-painted. The metal will appear splattered and may have rust or rust stains from the iron or steel base metal if it has been galvanized. Steel and galvanized iron are both magnetic Zinc that has been cast or pressed will seem grayish-white in color. A magnet won't stick to pure zinc since it is not magnetic. A painted sheet metal feature's composition—zinc, galvanized iron, or steel—can also be determined via a magnet test. Steel and galvanized iron are both magnetic, while pure zinc is not. Common Uses In the past, galvanized steel and iron were frequently used for: Cornices and additional wall decor window and door hoods Pantiles and decorative shaped shingles that mimic other materials ornamental crestings and finials for roofs Today's common usage include: Sheet metal is used for gutters, downspouts, and flashing. nails made of hot-dip galvanized steel.
Metallic spraying is when molten zinc is applied in a fine mist to a clean iron or steel part. To create an alloy, the coating can then be heated and fused with the surface of the iron or steel. Benefits: Coating produces products that are less fragile than those made by some of the other techniques. On bending, the coating won't peel or flake. Limitations: As corrosion agents fill in the pores, the covering becomes more porous and eventually impermeable. Painting: As a protective coating, paint incorporating zinc dust pigments may be used. Advantages Advantages In situ painting is a possibility. Limitations: Compared to the other zinc coating techniques mentioned above, this one is less successful. Pure zinc, galvanized steel, or iron do not take paint well. When paint on galvanized iron and steel peels, it typically removes completely, leaving a spotless metal surface exposed. It might be challenging to tell whether sheet metal components are made of zinc, galvanized iron, or steel if they are well-painted. The metal will appear splattered and may have rust or rust stains from the iron or steel base metal if it has been galvanized. Steel and galvanized iron are both magnetic Zinc that has been cast or pressed will seem grayish-white in color. A magnet won't stick to pure zinc since it is not magnetic. A painted sheet metal feature's composition—zinc, galvanized iron, or steel—can also be determined via a magnet test. Steel and galvanized iron are both magnetic, while pure zinc is not. Common Uses In the past, galvanized steel and iron were frequently used for: Cornices and additional wall decor window and door hoods Pantiles and decorative shaped shingles that mimic other materials ornamental crestings and finials for roofs Today's common usage include: Sheet metal is used for gutters, downspouts, and flashing. nails made of hot-dip galvanized steel. 
galvanized iron sheet thickness
Galvanized sheets' gauges are determined by their thickness, and the gauges make it clear for what projects the product is suitable. One of the most widely used forms of steel is galvanized sheet metal. This is as a result of its improved durability, formability, and strength. Its utility is significantly increased by the corrosion-resistant quality. Due to its adaptability, it can be used for a wide range of purposes. Utilizing galvanized steel has a lot of benefits as well. It's necessary to comprehend the underlying principle of galvanization before we proceed with that. How Does Galvanization Offer Base Metal Protection? The fundamental idea behind galvanization is to permanently join steel with zinc to produce a base metal that is more resistant to rust. Galvanization comes in many different forms, each with its own distinct principles. However, galvanization often operates according to the following tenets: Acid and other corrosive chemicals are kept from accessing the base metal by the zinc coating. When the coating is damaged, zinc compromises its anode more frequently than the base metal. As a result, it provides total rust protection. More quickly than the basic metal, zinc corrodes. The basic metal is well-protected by this activity. Chromate may be used because the goal is to let the zinc rust before the metal to provide appropriate protection.  However, pretreatment and posttreatment are quite important for a successful galvanization procedure. Ineffective pretreatment prevents the molten zinc from thoroughly reacting with the steel to form a flawless galvanized layer. Furthermore, the appearance of the galvanized film will be harmed by an ineffective post-treatment procedure. Consequently, components' value is decreased. For the purpose of preventing corrosion, there are several different types of galvanization. Every one of these categories performs and behaves differently. The many kinds of galvanizations are briefly described in the paragraphs that follow. Galvanization in the hot dip The most used galvanization technique is hot-dip galvanization. It entails dipping the steel in molten zinc, as the name would imply. About 860°F (460 °C) is frequently maintained by the pool of molten zinc. The base metal is properly chemically cleaned before being fluxed to remove any remaining oxides. A metallurgical link between the zinc and the target metal starts to form. After removing the metal from the bath, pure zinc reacts with atmospheric oxygen to create zinc oxide. After that, the zinc oxide and carbon dioxide combine to create zinc carbonate. The final coating on the base metal is made of zinc carbonate. Typically, the surface of this type of galvanized metal has a crystalline-like pattern. It is a straightforward and affordable option. Galvanization by electricity This type doesn't employ a molten zinc bath, in contrast to the hot-dip process. Instead, the zinc ions are transferred into the metal via an electrical current in an electrolyte solution. Electrical reduction and deposition of the positively charged zinc ions on the charged substance. The precise and homogeneous coating thickness is a benefit. Galvannealing Galvannealing combines the annealing and hot-dip galvanization processes. This process results in galvanized steel with a unique coating. Instantaneous hot dipping and annealing take place. As a result, you eagerly anticipate creating a matte gray finish. This process results in galvanized sheet metal that can survive welding and is good for adhering paint.
However, pretreatment and posttreatment are quite important for a successful galvanization procedure. Ineffective pretreatment prevents the molten zinc from thoroughly reacting with the steel to form a flawless galvanized layer. Furthermore, the appearance of the galvanized film will be harmed by an ineffective post-treatment procedure. Consequently, components' value is decreased. For the purpose of preventing corrosion, there are several different types of galvanization. Every one of these categories performs and behaves differently. The many kinds of galvanizations are briefly described in the paragraphs that follow. Galvanization in the hot dip The most used galvanization technique is hot-dip galvanization. It entails dipping the steel in molten zinc, as the name would imply. About 860°F (460 °C) is frequently maintained by the pool of molten zinc. The base metal is properly chemically cleaned before being fluxed to remove any remaining oxides. A metallurgical link between the zinc and the target metal starts to form. After removing the metal from the bath, pure zinc reacts with atmospheric oxygen to create zinc oxide. After that, the zinc oxide and carbon dioxide combine to create zinc carbonate. The final coating on the base metal is made of zinc carbonate. Typically, the surface of this type of galvanized metal has a crystalline-like pattern. It is a straightforward and affordable option. Galvanization by electricity This type doesn't employ a molten zinc bath, in contrast to the hot-dip process. Instead, the zinc ions are transferred into the metal via an electrical current in an electrolyte solution. Electrical reduction and deposition of the positively charged zinc ions on the charged substance. The precise and homogeneous coating thickness is a benefit. Galvannealing Galvannealing combines the annealing and hot-dip galvanization processes. This process results in galvanized steel with a unique coating. Instantaneous hot dipping and annealing take place. As a result, you eagerly anticipate creating a matte gray finish. This process results in galvanized sheet metal that can survive welding and is good for adhering paint. 
galvanized iron sheet coated
Galvanized iron sheet is coated with zinc to make the surface resistant to corrosion and rusting. The previously mentioned zinc coating weights of galvanized steel are managed by steel mills during the production cycle. These zinc coating weights can vary based on the requirements of the customer as well as the product's final end use. The predicted length of service life of the steel is directly proportionate to the coating weights that are applied. Zinc coating weights range from G90 to G60, G40, and G30 galvanized steel and are measured in ounces per square foot. Because it has a heavier coating weight of zinc applied to it, G90 galvanized steel has a longer service life than G60, G40, and G30 galvanized steel. This is because the coating weight of zinc applied to G90 galvanized steel is 0.90 oz/ft2, whereas the coating weight of zinc applied to G60 galvanized steel is.060 ox/ft2, and so on.  The use of galvanized steel has a number of benefits, including a relatively low cost in comparison to other goods, such as stainless steel, a long life duration, low maintenance requirements, and the ability to be produced fast. Because of this, galvanized steel is utilized in the production of thousands of products that are all around us and that we utilize on a daily basis. The industries of heating, ventilation, and air conditioning (HVAC), construction, roofing, appliance manufacturing, waste management, and container manufacturing, to name a few, all make use of hot-dipped galvanized steel. For instance, in the heating, ventilation, and air conditioning (HVAC) industry, galvanized steel sheet, coil, and slit coil (with widths such as 5.394 inches) are utilized in the production of duct and spiral ducts. It is possible to pre-paint galvanized steel, to paint it after it has been galvanized, or to leave it unpainted.
The use of galvanized steel has a number of benefits, including a relatively low cost in comparison to other goods, such as stainless steel, a long life duration, low maintenance requirements, and the ability to be produced fast. Because of this, galvanized steel is utilized in the production of thousands of products that are all around us and that we utilize on a daily basis. The industries of heating, ventilation, and air conditioning (HVAC), construction, roofing, appliance manufacturing, waste management, and container manufacturing, to name a few, all make use of hot-dipped galvanized steel. For instance, in the heating, ventilation, and air conditioning (HVAC) industry, galvanized steel sheet, coil, and slit coil (with widths such as 5.394 inches) are utilized in the production of duct and spiral ducts. It is possible to pre-paint galvanized steel, to paint it after it has been galvanized, or to leave it unpainted.

0
0